Explore Mechanism, Features, Advantages, Applications of Electro Oxidation Processes
Electro Oxidation Wastewater Treatment
Brief Introduction About Electro Oxidation Wastewater Treatment
We discussed about the mechanisms of electro oxidation wastewater treatment processes, by explaining direct and indirect oxidation, and how these two mechanisms enhance the comprehensive capability and efficiency of refractory organic compounds degradation, mineralization, and explore what the factors impact the treatment efficiency and performances of electro oxidation wastewater treatment processes.

Electro oxidation is one of most innovative and advanced wastewater treatment techniques amongst advanced electro oxidation processes (AEOs) adopting anodic oxidation or electrochemical advanced oxidation that apply electricity to mineralize refractory organic contaminants.
Electro oxidation technology requires electrochemical cell setup, electrodes, electricity input, and electro oxidation/reduction reactions within the electrolysis processes.Electro oxidation technology are applied to treat industrial effluents, or improve biodegradeability of complex wastewater..
The Mechanism Of Electro Oxidation Wastewater Treatment
Electro oxidation requires applying electrical energy, electron trafer between the electrolyte, and anode was expedited once electricity applied, certain organic pollutants could be mineralized directly around the surface while electron transfer processes happen between the anode and electrolyte, that is the mechanism of direct oxidation within electro oxidation processes.
Indirect oxidation invloves generation of oxidizing agents such as hydroxyl radicals (OH), with higher oxidation potential amongst its types and other highly active radicals, for instance, sulfate radicals (SO) within the electrolytic reaction processes, migrate into the solution. Radicals mineralize organic matters by breaking chemical bonds such as C-C, C-N, and etc, and degrade into ingranic forms (H2O, CO2, etc,) or simpler organic compounds as intermediates with smaller molecules could be mineralized with further oxidation via a vast variety of pathways, and eventually degraded, instead of concentrated, does not for further treatment afterwards.
Direct And Indirect Oxidation Processes for Treatment Of Wastewater Via BDD Electrolysis

Introduced in 1970, electrochemical oxidation technology has gained significant attention in wastewater treatment and disinfection due to its environmentally friendly and efficient characteristics. When coupled with flocculation, biochemistry, membrane treatment, and other technologies, it offers remarkable advantages and promising prospects for complete removal and mineralization of reclatriant organic compounds from wastewater.
Utilizing BDD as the electrode anode material, electrochemical oxidation technology can effectively degrade organic compounds through direct and indirect oxidation processes. Direct oxidation involves the removal of organic matter by adsorbing organic pollutants onto the anode surface through electron transfer. This process can further be categorized into electrochemical conversion and electrochemical combustion based on the degree of oxidation.

Indirect oxidation, on the other hand, involves the removal of organic pollutants by generating active intermediates and highly active oxidizing agents (oxidants) susch as hydroxyl radicals, hydrogen peroxide, and other reactive species on the anode surface and then the surrounding areas. These oxidants can mineralize organic compounds into different types of intermediates, and eventually break them into water and inorganic matters.
Indirect oxidation processes is one of the major mechanisms with electro oxidation treatment of wastewater, and it’s critical for treatment of different types of complex wastewater and disinfection of water, therefore it’s critical to choose an electrode material that is physically and chemicallu stable, resistant to corrosion, endure constant operation in different current densities, voltages and various types of electrolyte, less chance of fouling.
Boromond leverages electro oxidation wastewater treatment modular units to coduct trial scale wastewater treatability testing, on-site complex wastewater treatment, up to pilot scale industrial effluent treatment and water remediation based on BDD electrode material as the basic electrode material, and years of experiences with electrolytic wastewater treatment, electrochemical advanced oxidation processes (EAOPs) technology to powering consistent and constant mineralization of reclatriant organic pollutants under normal temperature and pressure conditions. This electro oxidation treatment processes reduces the additionals of toxic chemicals, relying solely on electricity consumption with minimal material usage and maintenance.
All these sustainable and highly efficient electro oxidation wastewater treatment solutions that contribute to effective and environmentally-friendly wastewater management, thanks to bulk generation of active oxidants/oxidizing agents, which lead to massive mineralization of refractory organic compounds, so electro oxidation wastewater treatment technology could play a critical role in treatment of complex wastewater.

Water molecule electorlysis processes
Water, being a polar molecule, exhibits a positive and negative end. During the process of water electrolysis, the positively charged hydrogen atoms are attracted to the negatively charged electrode (cathode), while the negatively charged oxygen atom is drawn towards the positively charged electrode (anode). As a result, the water molecules dissociate into their respective ions: hydrogen ions (H+) at the cathode and hydroxide ions (OH-) at the anode.
The water electrolysis process can be summarized by the following half-reactions:
At the cathode: 2H+ + 2e- -> H2 (hydrogen gas)
At the anode: O2 + 2H2O + 4e- -> 4OH- (oxygen gas)
Main Factors Affect Electro Oxidation Wastewater Treatment Efficiency
In this part, we will cover main factors affect electro oxidation wastewater treatment efficiency, mainly based on our years of experiences with electro oxidation treatablity test, wastewater treatment product research and development processes, and implement of electro oxidation wastewater treatment products, from trial scale, to pilot scale industrial wastewater treatment projects. Check the key points affect electro oxidation wastewater treatment efficiency we listed below:
Electrode Materials
People in water and wastewater treatment industry and electrochemical research niche have adopted a vast variety of electrode materials for electro oxidation wastewater treatment processes, factors such as:
Capability of electrochemical generation of oxidizing agents, which affects the comprehensive organic compounds removal efficiency as different electrode has different surface property and electro-catalytic activity.
Chemical and mechanical robust, electrodes with stable chemical and mechanical stability can emit constant and stable performance within the electrolytic processes, and that means less chance of fouling.
Stainless steel, platinum, lead dioxide (PbO2), mixed metal oxide(MMO), titanium, and boron-doped diamond BDD electrode and etc, are adopted to conduct electro oxidation wastewater treatment, we will resume dicussions about comparison of different electrode materials in electro oxidation application in other contents.
Surface Areas
Actually electro oxidation reaction efficiency will be affected by the surface area of an electrode, as active area of electrodes, electron transfer, and larger surface area means enhanced electro oxidation of refractory organic compounds within the electrolyte (wastewater), therefore improve the comprehensive oxidation efficiency, yes, as you may have noticed from the previous section, surface area is one of those factors impact direct and indirect oxidation.
Wastewater Compositions
Characteristics of wastewater such as oxidation potentials, potential to be degraded into intermediates, or inorganic matters, are different amongst different type of organic pollutants, and ions in the waste streams might affect the conductivity of the electrolytes, therefore it's critical to conduct treatability testing prior to electro oxidation technology implement in actual wastewater treatment, that is to identify the content of the wastewater you need treat, via electro oxidation, and it's also immportant to find an approach to meet your treatment objectives.
pH Level
Hydroxyl radicals are the major group of reactive oxygen species mineralize organic compounds within the electro oxidation processes, and yes, pH level within the electrolyte (wastewater) does affect the electrochemical generation of these active oxidizing agents on the surface of anode, as hydroxyl radicals will be more prevailing in alkaline conditions, according to our tests.
Current Density
Current density reflects the mass transfer of electron, which means current density could actually affect the efficiency of direct oxidation, transfer of electrode, therefore it's critical to keep some mediate current density to expedited oxidation reaction rate, but yet, minimize chances of undesired oxygen evolution, that is the key to constant electro generation of reactive oxygen species.
Electrochemical Cell Design
Electrochemical cells are critical when it comes to implements of electro oxidation wastewater treatment processes, as electrochemical cells are the main spaces for electrolytic processes, and distance between electrodes, active electrode surface area, cell holding space, flow rate, initial COD level, hydraulic retention time, and COD removal efficiency.
Electro Oxidation Wastewater Treatment Explained In Flow Diagram And Implements
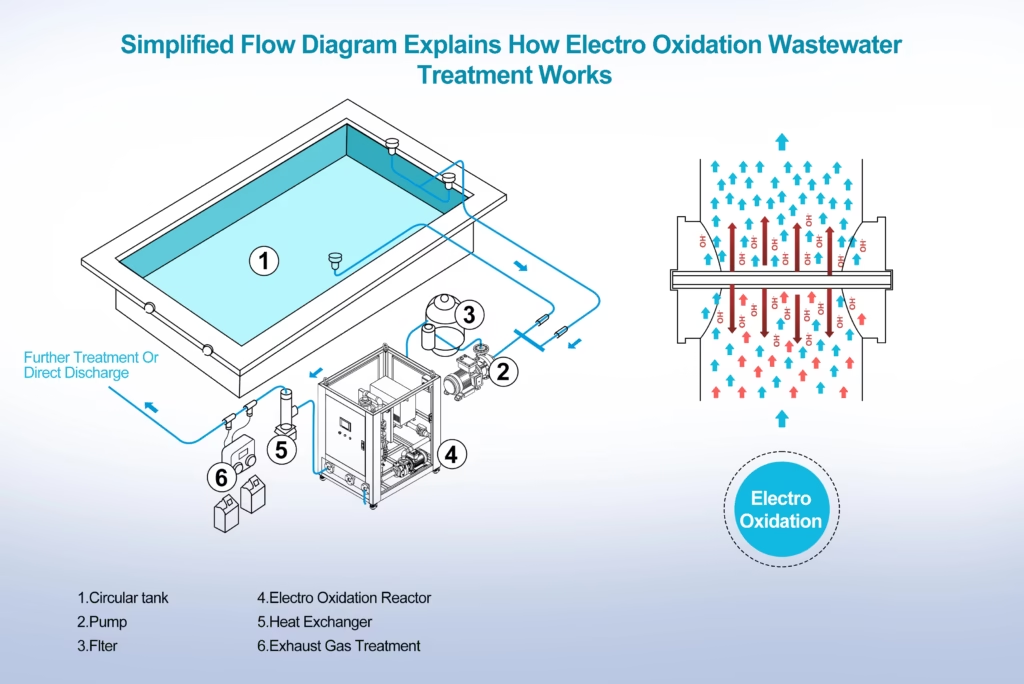
Here at Boromond, we are dedicated to offer carbon based boron doped diamond BDD electrode with expanded surface area, proper boron doping level, Sp2/Sp3 structures, and surface termination to make catalyst electrode materials favor electrochemical oxidation by expediting reactive oxidants generation.
Eelectrolytic cells with boron doped diamond electrodes as anodes and basic components are precisely configured to be flow-through to boost wastewater treatment efficiency and decrease retention time, enhanced peformance at organic pollutants removal.
Our electrochemical oxidation wastewater treatment products, solutions are ready to be roll, all you need to do is to tell us your wastewater compositions, budgets and required discharge standards.
Electro Oxidation Wastewater Treatment Features & Advantages
Environmental friendly: Redox reagents used to mineralize organic pollutants anre electrochemically generated during the wastewater electrolysis processes,which means electro oxidation doesn’t require the addition of toxic chemicals.
Efficiency: Electro oxidation is highly efficient when it comes to removal of various types of organic compounds found in waste streams from different industries.
Easy operation and automate: Given its mediate requirement toward operation conditions, ease to add automatic operator access, electro oxidation is a great option for complex wastewater treatment projects.
Less chance of sludge generation: Unlike other advanced oxidation processes (AOPs) such as Potassium permanganate oxidation: and Fenton processes (an Iron-based AOPs treatment method), electro oxidation has no or few byproducts when it comes to sludge formation.
Modular design and scalability: Electro oxidation wastewater treatment equipments and systems are designed to be scalable and all electro-oxidation systems are modular treatment units, and ready to custom to meet your application needs.
Electro Oxidation Wastewater Treatment Application Ranges
Electro oxidation wastewater treatment methods could be applied to treat industrial wastewater with refractory organic pollutants at high concentration level.
Wastewater with different pH level (except those with fluoride ion)
Organic wastewater with high salinity, higher salt content means higher conductivity
Further treatment of concentrate after membrane, MVR, RO
Wastewater contain toxic CN-, NaN3, organophosphorus, organic sulfur
Various types of water defined as hazardous waste streams
Improve effluents quality, COD level to compliance with regulations
Organic wastewater with organic pollutants can not treated via standard biological treatment methods.
Electro Oxidation Wastewater Treatment Results & Pollutant Removal Efficiency
Check the charts below to find out treatment efficiency and results of electro oxidation wastewater treatment technology, share your water profile to get an assessment over your industrial effluents, it’s free of charge, we offer not only treatment methods, but also a comprehensive solution, service to various industries.






Send your water profile to get a free water profile analysis from a wastewater treatment expert now, treatability checking right away.
Why Boromond For Electro Oxidation Wastewater Treatment
Innovative
Innovation is always a critical part of our engineering, technical and implements practices within the journey to seek better electro oxidation wastewater treatment products, service and solution.
Cooperation
It's critical to listen and comprehend what our customers need all the time, especially when it comes to meet treatment objectives, enhance treatment processes, improve treatment effciency and reduce maintenance.
Paractical
We are always here to share first-hand information about different waste streams, and how we find out the right pathways and solution to treat these complex wastewater effectively while meet your specific needs.
Electro Oxidation Wastewater Treatment Projects & Case Studies
By delving into these case studies and projects, we provide abundant data and information on the successful application of BDD electrodes and electro oxidation wastewater treatment systems across diverse industries. We always deliver solution with sustainability, environmental stewardship, and economic viability through innovative water treatment technology. Join us in revolutionizing the way we approach wastewater treatment, one case study at a time.

Check Cases of Pharmaceutical Wastewater Treatment, And How BDD Electrode Remove COD, BOD etc

BDD Eletrodes and Industry Scale Modules Are Used To Eliminate Complex In Wastewater Treatment Process.

Explore Case Studies On Degradation Of Refractory Organic Compounds In Oil & Gas Industry

Find Out How Boromond Discover Methods To Degrade Food Processing Wastewater with High COD,BOD and SS.

Boromond Managed To BDD Treat Organic Wastewater From Pesticide Production,Click To Explore

Click To Disclose How BDD Electrodes Degrade Organic Pollutants from Textile Industry Wastewater
Electro Oxidation Wastewater Treatment Solutions
Explore electro oxidation wastewater treatment solutions designed to solve problems with complex wastewater from different manufacturing sectors and find out how we can help you to tackle various types of challenges with innovative electro oxidation wastewater treatment products, services, and years of experiences with previous and ongoing projects.
Electro oxidation are applied to treat various wastewater from pharmaceutical and personal cares manufacturers
Implements of EO technology to treat effluents originated from petrochemical, oil and gas industry
Remove recalcitrant organic compounds from wastewater generated within LIB production and recycling processes
Engineering Design Services Based On Electro Oxidation Wastewater Treatment

Maximum Treatment Efficiency
Engineered to enhance removal rate of various organic pollutants , and maximum treatment efficiency toward complex wastewater, it’s the time to figure out how our engineering design locate the right pathways to solve your problems.

Full Range Services
We offer full ranges of services relevant to electro oxidation wastewater treatment processes that mainly encompasses design, fabrication, deployments, installation, operation and maintenance so you can focus on your sole target: meeting treatment objective

Value-Added Service
Start with synthesis of catalyst electrode materials, and fabrication of electro oxidation wastewater treatment systems, up to optimal engineering solution, and treatment system integration, we offer valuable services based on expertise, years of experiences.
More questions about electro oxidation wastewater treatment? reaching out now.
We offer free consulations and evaluations about the treatability and application of electro oxidation wastewater treatment technology toward your water, all you need to do is leaving a message to us mentioning details of your water profile.

