Electro Oxidation Basics: industrial-scale hydroxyl radicalS generation via BDD electrode
Engineering Hydroxyl Radicals Flux with BDD Electrode
Right after we get in-depth comprehension over the function of BDD electrode in electro oxidation wastewater electrolysis processes within the BDD electrode wastewater electrolysis, in this context, we introduce, hydroxyl radicals generation via BDD electrodes, a critical part of electro oxidation wastewater treatment process which is growingly acknowledge as an efficient and effective alternative to conventional biologival, advanced oxidation process (AOP), or physico-chemical treatment methods for the treatment of wastewater containing refractory organic pollutants.
Meanwhile electro oxidation process is not only about degradation and mineralizations of recalcitrant organic compounds, but also removing non-biodegradable or inhibitory compounds, handling high salinity or even toxic waste streams, capability to function well and reach treatment objectives in implements that traditional AOPs are less efficient or require more reagents.
What is Hydroxyl Radical? How Does This Radical Work? Explore How Hydroxyl Radical Functions In The Electro Oxidation Wastewater Treatment
In this part, we introduce the basic information of hydroxyl radical, and how does this type of radical works, and what is the role of hydroxyl radical in electro oxidation wastewater treatment processes, that is the machanisms and pathways of indirect oxidation process that mineralize a wide range of recalcitrant organic compounds in complex wastewater.

Understanding Hydroxyl Radicals (•OH)
Hydroxyl •OH radicals are molecules made of one oxygen and one hydrogen atom, with an unpaired electron, make them highly reactive and unstable.
They form naturally in the atmosphere, water, and inside cells, hydroxyl radical are often generate through reactions with UV light or adding certain chemicals in conventional wastewater treatment methods.
With a molecular weight at some 17.007 g/mol, OH radicals are very reactive, they quickly attack nearby molecules.
•OH is non-selective therefore it can react with wider range of persistent organic compounds, usually causing rapid degradation and depolymerization, breaking chemical bonds of those recalcitrant organic molecules to intemediates or smaller molecules which can be further mineralized, sometimes simply inorganic matters.
Oxidation capability of hydroxyl •OH radical make it an important component in processes like breaking down organic pollutants or initiating chemical reactions, it plays an important role in the electro oxidation wastewater treatment processes, especially in indirect oxidation or say mediated oxidation process, that is mineralizations of various organic pollutants on the anode surface, associated with other reactive oxidizing agents.
Indirect Electro Oxidation Processes via Hydroxyl Radicals

Oxidation Capability of Hydroxyl Radicals
With an electrochemical oxidation potential at 2.8 V, hydroxyl •OH radical are come second place on major reactive oxidizing species and agents, with their unique highly potent, hydroxyl •OH radical are capable of supplying a speedy breaking down of organic compounds, as they react readily with and broadly conduct non-selective attack with almost all types organic molecules, initiating several recalcitrant organic compound degradation pathways that other reactive oxidants such as Ozone, hydrogen peroxide, hypochlorite, and chlorine can’t easily tackle, especially aromatic rings, for instance benzene rings, those organic pollutants could be mineralized into different intermediates for further degradation, and eventually mineralized into inorganic matters such as water and carbon dioxide.
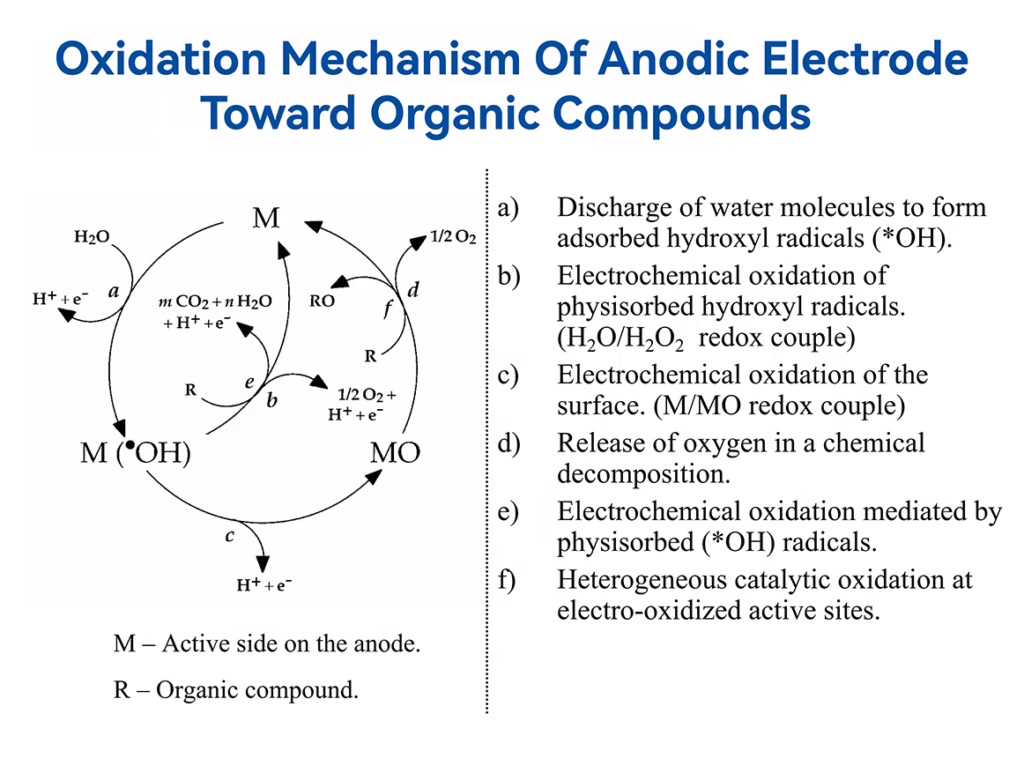
Hydroxyl Radicals & Indirect Oxidation
Indirect oxidation involves the removal of organic pollutants by reaction of reactive oxidizing agents such as hydroxyl radical, hydrogen peroxide, and other reactive species with organic pollutants around the anode surface.
As one of the major mechanisms in electro oxidation processes, indirect oxidation process has several mechanisms:
a). Discharge of water molecules to form adsorbed •OH radicals.
b). Electrochemical oxidation of physisorbed hydroxyl •OH radicals.
c). Electrochemical oxidation of the surface.
d). Release of oxygen in a chemical decomposition.
e).Electrochemical oxidation mediated by physisorbed (*OH) radicals.
f).Heterogeneous catalytic oxidation at electro-oxidized active sites.
Origin of The Radicals: Fundamental Mechanism of Hydroxyl Radicals Generation
As you may have noticed, rather than direct oxidation of organic pollutants on the anode surface, which is called direct oxidation process, electro oxidation uses indirect oxidation, that is applying electricity to split water molecules to form adsorbed hydroxyl radicals, that is water molecules loses an electron and a proton once it contacts a positively charged anode, therefore hydroxyl radical is formed:
H₂O → •OH+ H⁺ + e⁻
With an electrochemical oxidation potential second only to fluorine (check the Hydroxyl Radical & Indirect Oxidation section of this page), hydroxyl radical act as a “molecular wrecking ball”, they simply take electrons away from any organic pollutant they attacks, effectively “mineralizing” them.
Hydroxyl radical are mainly generated at the anode through the electrochemical oxidation of water molecules. Water discharge happens once the applied potential overruns the oxygen evolution doorstep, producing adsorbed hydroxyl radical on the electrode surface
The behaviors, reactivity, and fate of these radicals are rely strongly on the phyiscochemical properties of the anode material. Anodes are often categorized as active or non-active based on their interaction with hydroxyl radical. Non-active anodes, such as boron-doped diamond (BDD electrode), demonstrate less adsorption of •OH, allowing radicals to remain highly reactive and available for indirect oxidation of organic pollutants. In contrast, active anodes (e.g., Platinum, RuO₂-based mixed metal oxides) tend to form higher oxide layers, which participate in mediated oxidation pathways and curb accessibility of reactive hydroxyl radical, which eventually limits complete mineralization of organic pollutants.
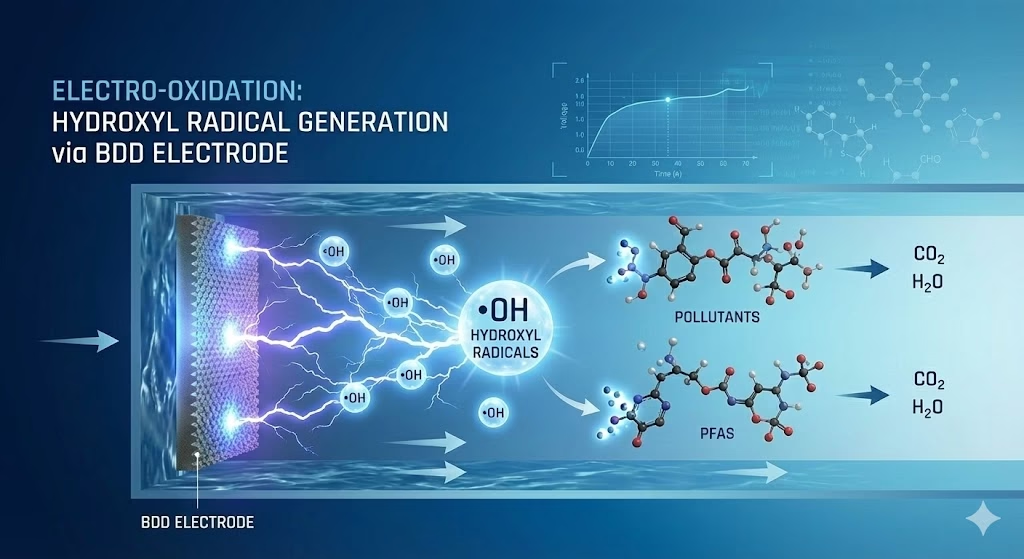
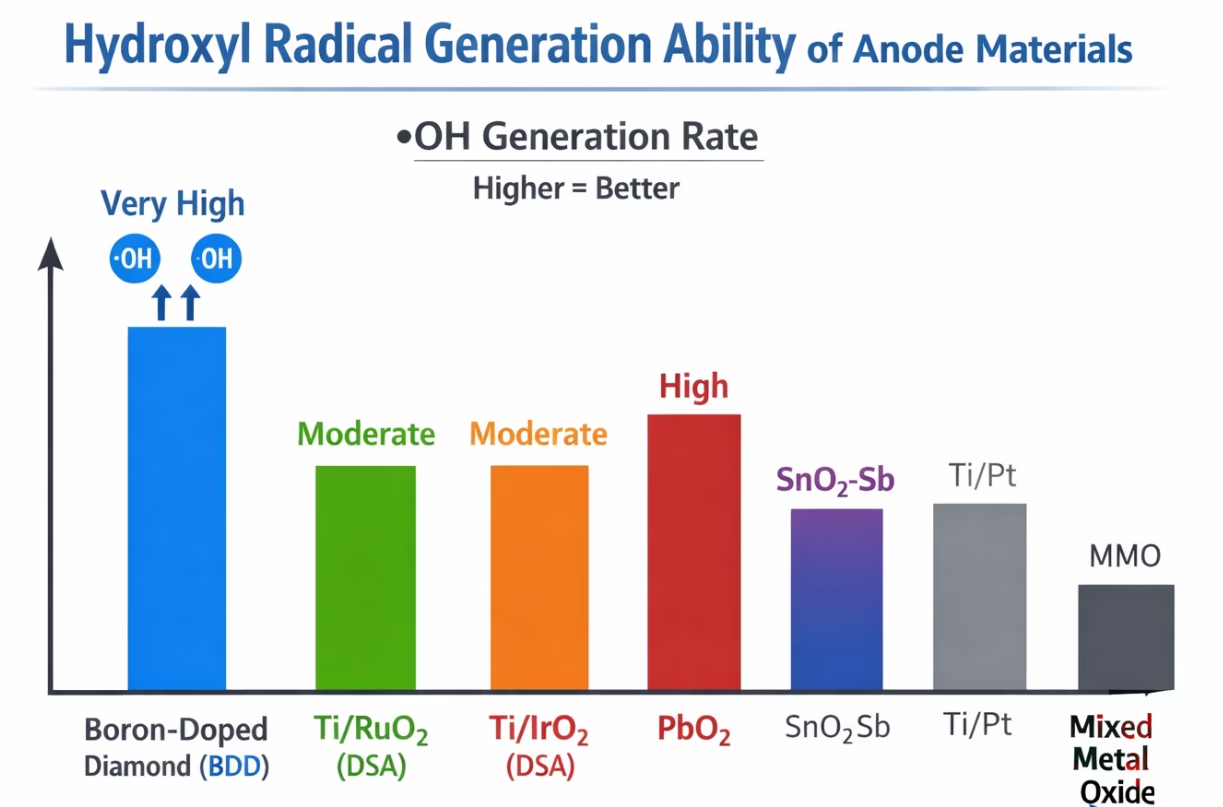
BDD electrode is efficient in hydroxyl radicals production than regular anodes.
Boron doped diamond BDD electrode, a type of non-active anodes, exhibit weak interaction with •OH, allowing radicals to retain their high oxidative potential, and unmatched capability to produce and maintain with high anodic potentials, and what is more, BDD electrodes demand a very high voltage to produce oxygen gas, which means most of the electrical energy goes into generating highly reactive hydroxyl radical (•OH) instead, making the process energy-efficient. it attracted specific attentions, check scientific research papers relevant to hydroxyl radicals production via bdd electrode. Check BDD electrode vs Ti/RuO2 anode

Why BDD Electrode for Electro Oxidation
Unlike conventional electrodes, surface of BDD anode doesn’t readily react with the generated hydroxyl radical, those oxidants are weakly adsorbed, which makes it possible to ensure these reactive species are remained highly reactive and diffuse into the bulk solution to oxidize all types of organic pollutants.
Unique sp3-hybridized carbon structures offer resistance to corrosion and way less chance of electrode fouling, enable constant enactment in harsh environment, essential for effective and sustainable operation over commercial scale wastewater treatment applications under versatile voltage range, waste stream compositions, and different persistent organic pollutants.
A Comparison of Different Electrodes On Hydroxyl Radicals Generation, More Data
COD removal efficiency and color removal rate help us to get a better understanding of differences between BDD electrodes and conventional electrodes.



More questions about electro oxidation wastewater treatment? reaching out now.
We offer free consulations and evaluations about the treatability and application of electro oxidation wastewater treatment technology toward your water, all you need to do is leaving a message to us mentioning details of your water profile.
