Electro Oxidation Basics#2: Why anode Selection matters IN hydroxyl radicalS generation & EO Treatment
A Comparison of BDD Electrode Vs Ti RuO₂ Anode, A Typical Mixed Metal Oxide Anodes in Electro Oxidation Wastewater Treatment
When comparing Boron-Doped Diamond BDD electrode and Dimensionally Stable Anodes (DSA) like RuO2 anode, a thin electrode film with Ruthenium Dioxide coating on a titanium substrate, we are looking at two fundamentally different philosophies of oxidation.
In the field of electrochemical advanced oxidation processes (EAOPs) or simply electro oxidation, the anode is not a passive component, the choice of anode material isn’t just a matter of durability as it dictates the very chemistry of how water molecules are initiated, which kinds of oxidizing agent really functions, whether organic pollutants are lightly tranformed remodel or completely mineralized.
Among all commercially relevant anode materials, boron‑doped diamond BDD electrode and Ti/RuO₂ (DSA‑type) anodes sit at opposite ends of the electrochemical spectrum. They can both be used for electro oxidation processes, but they do so in fundamentally different ways of doing oxidation.
This page is all about comprehending these differences between boron doped diamond BDD electrode and RuO2 anode, therefore we sort the contents into two separate parts. First, we look at how BDD electode and Ti RuO₂ anode generate and handle hydroxyl radicals at the anode surface, which is core part of indirect oxidation process that is one of the two basic mechanisms of electro oxidation processes, and essential elements for designing electro oxidation treatment processes that actually work as anticipated, then we dive deeper to explore and discuss how do BDD electrode and Ti Ru02 differentiate acutal electro oxidation processes.
The Core Divergence: "Active" Ti RuO2 Anode vs. "Non-Active" BDD Anode
In this part, we explore hydroxyl radicals generation or say oxidation mechanisms of BDD electrode and Ti/RuO2 anode, dive deeper to figure out the differences between non-active anode and active anode, why some electrode materials perform better than others when it comes to the basics of electro oxidation wastewater treatment processes, which also help you to get a better comprehension about the anode material selection part.

Hydroxyl •OH radicals are molecules made of one oxygen and one hydrogen atom, with an unpaired electron, make them highly reactive and unstable.
They form naturally in the atmosphere, water, and inside cells, hydroxyl radical are often generate through reactions with UV light or adding certain chemicals in conventional wastewater treatment methods.
With a molecular weight at some 17.007 g/mol, OH radicals are very reactive, they quickly attack nearby molecules.
•OH is non-selective therefore it can react with wider range of persistent organic compounds, usually causing rapid degradation and depolymerization, breaking chemical bonds of those recalcitrant organic molecules to intemediates or smaller molecules which can be further mineralized, sometimes simply inorganic matters.
Oxidation capability of hydroxyl •OH radical make it an important component in processes like breaking down organic pollutants or initiating chemical reactions, it plays an important role in the electro oxidation wastewater treatment processes, especially in indirect oxidation or say mediated oxidation process, that is mineralizations of various organic pollutants on the anode surface, associated with other reactive oxidizing agents.

•OH Radicals Generation On RuO2 Anode
In electrochemistry, we classify anodes based on how they interact with the hydroxyl radical formed during water discharge.
Ti/RuO2 anode, a typical active anode, has a high affinity for oxygen. The •OH radicals formed are chemisorbed to the surface, effectively becoming part of the oxide lattice.
Oxide lattices, also named oxide lattice oxygen, it’s a group of oxygen atoms within the crystal structure of active anode material like Ruo2, oxide lattice reacts directly with contaminants, generating oxygen vacancies (OV).
These oxygen vacancies are subsequently replenished by water molecules and Gas-phase oxygen, which regenerates the oxide surfaceusually, that is the the Mars-van Krevelen mechanism, end up forming higher-state oxides or say direct oxide lattice oxygen oxidation.
RuO2(OH)→ RuO3 + H+ + e –
With a lower OEP at some 1.4–1.7V, RuO2 convert these radicals into a higher oxide or evolves into O2 gas. This “active” oxygen then attacks specific functional groups in organic molecules, accomplish effective mineralizations toward specific organic compounds but typically leads to lower overall mineralization efficiency towards persistent organic pollutants, that is why we call it selective.
The generation of these radicals follows a similar initial step, but their fate differs wildly depending on the electrode’s Oxygen Evolution Potential (OEP).
The Formation Step: Both electrodes initiate oxidation via the discharge of water: M+H2O → M(OH)ads+ H+ +e-, however electrochemical half-reaction step happened in various processes such as water electrolysis, corrosion, or electrocatalytic reactions, e.g., hydrogen evolution reaction (HER), or oxygen evolution reaction, OER).
With oxygen evolution potential at some 2.3 to 2.7V, BDD electrode has a much higher voltage threshold for the oxygen evolution reaction (OER), which critical for electro oxidation processes for wastewater treatment, as this will mitigate the undesired energy-consuming side reaction, aka water splitting processes, that is transfer water molecules into oxygen (OER), therefore focus on main target, bulk generation of highly reactive hydroxyl radicals to degrade organic pollutants.
BDD(OH) + Organic Compounds → BDD + CO2 + H2O + Inorganic Salts
While BDD electrode as a non-active anode, has weak interaction with hydroxyl radicals, reactive radicals remain physisorbed, essentially “free” to roam the near-anode surface with their full oxidation potential at 2.80 V vs. SHE. Leads to speedy degradation and complete mineralization of almost all type of recalcitrant organic pollutants.
BDD Electrode vs. RuO2 Anode in Practical Wastewater Treatment
In this part, we explore hydroxyl radicals generation or say oxidation mechanisms of BDD electrode and Ti/RuO2 anode, dive deeper to figure out the differences between non-active anode and active anode, why some electrode materials perform better than others when it comes to the basics of electro oxidation wastewater treatment processes, which also help you to get a better comprehension about the anode material selection part.
As you may have noticed, rather than direct oxidation of organic pollutants on the anode surface, which is called direct oxidation process, electro oxidation uses indirect oxidation, that is applying electricity to split water molecules to form adsorbed hydroxyl radicals, that is water molecules loses an electron and a proton once it contacts a positively charged anode, therefore hydroxyl radical is formed:
H₂O → •OH+ H⁺ + e⁻
With an electrochemical oxidation potential second only to fluorine (check the Hydroxyl Radical & Indirect Oxidation section of this page), hydroxyl radical act as a “molecular wrecking ball”, they simply take electrons away from any organic pollutant they attacks, effectively “mineralizing” them.
Hydroxyl radical are mainly generated at the anode through the electrochemical oxidation of water molecules. Water discharge happens once the applied potential overruns the oxygen evolution doorstep, producing adsorbed hydroxyl radical on the electrode surface
The behaviors, reactivity, and fate of these radicals are rely strongly on the phyiscochemical properties of the anode material. Anodes are often categorized as active or non-active based on their interaction with hydroxyl radical. Non-active anodes, such as boron-doped diamond (BDD electrode), demonstrate less adsorption of •OH, allowing radicals to remain highly reactive and available for indirect oxidation of organic pollutants. In contrast, active anodes (e.g., Platinum, RuO₂-based mixed metal oxides) tend to form higher oxide layers, which participate in mediated oxidation pathways and curb accessibility of reactive hydroxyl radical, which eventually limits complete mineralization of organic pollutants.
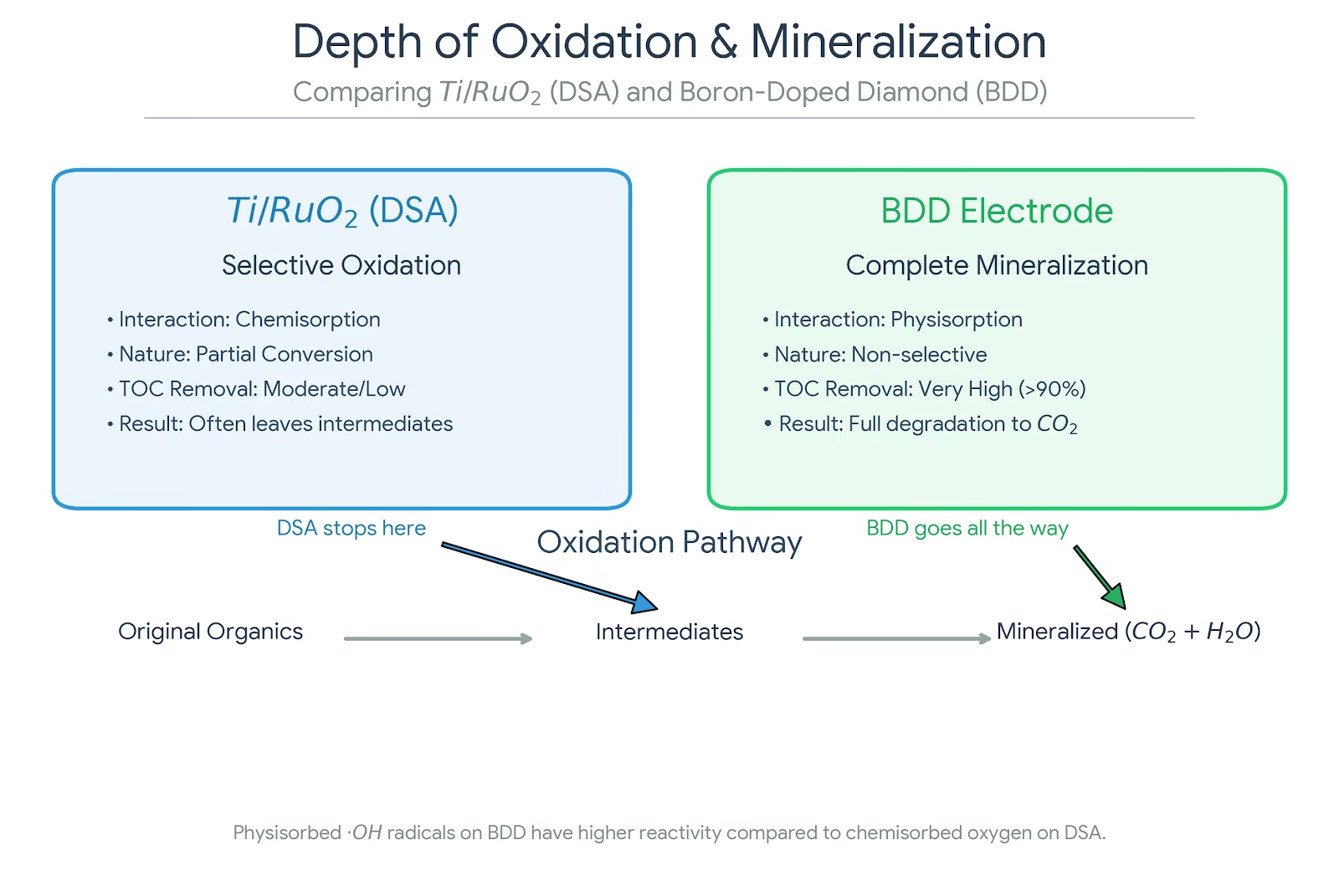
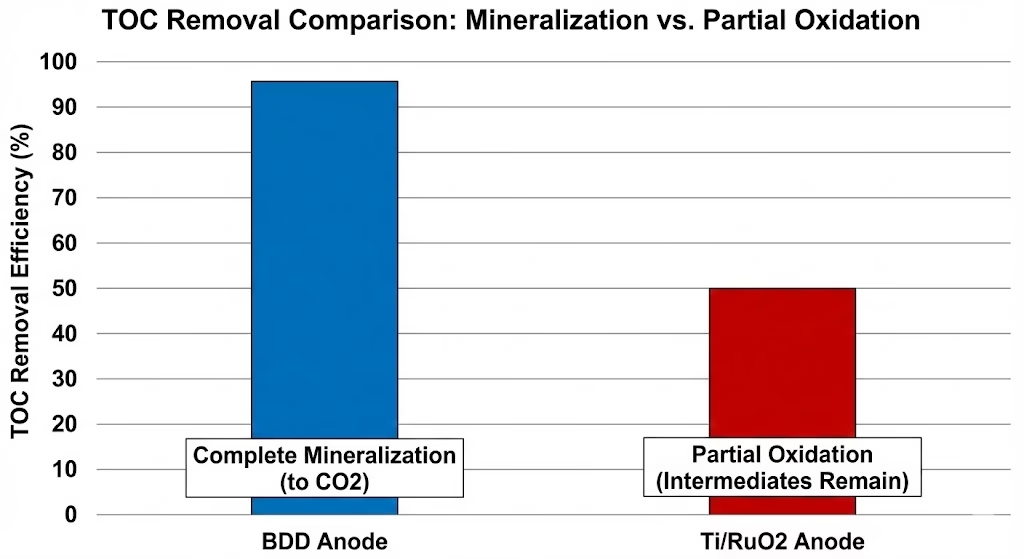
Depth of Oxidation and Mineralization
With BDD anodes, oxidation tends to go deep. Persistent organics, pharmaceuticals, dyes, and other refractory compounds are progressively broken down until little remains but CO₂ and inorganic ions. Stable intermediates are less likely to accumulate because the radicals driving the reaction are simply too reactive.
With Ti/RuO₂ anodes, oxidation is often partial. Functional groups are modified, color is removed, toxicity may drop—but complete mineralization is harder to achieve, especially for complex or highly stable molecules.
Neither outcome is inherently right or wrong; it depends entirely on the treatment objective.
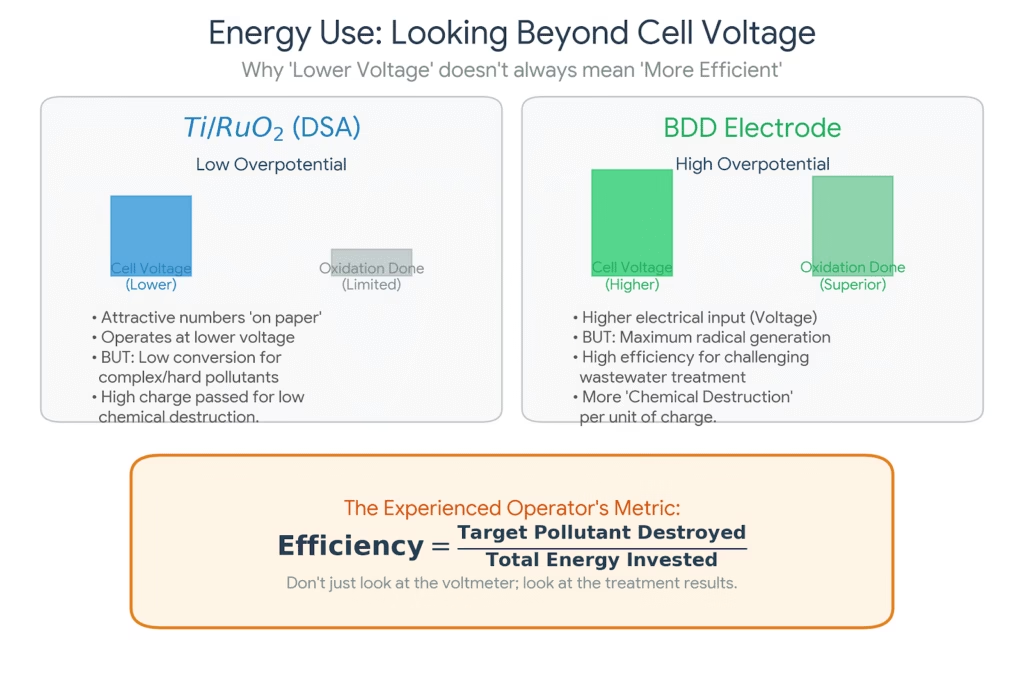
What Is Beyond Energy, Eyes On The EO Treatment Result
It is true that Ti/RuO₂ anodes operate at lower overpotentials and therefore lower cell voltages. In large, continuous systems, this translates into attractive energy numbers on paper.
BDD systems, by contrast, run at higher voltages. However, when the target pollutants are difficult to oxidize, BDD electrode often delivers more useful oxidation per unit of charge passed. For challenging wastewaters, the relevant metric is not voltage alone, but how effectively that electrical energy is converted into chemical destruction.
Experienced operators quickly learn that energy efficiency must be evaluated against treatment goals, not just electrical parameters.
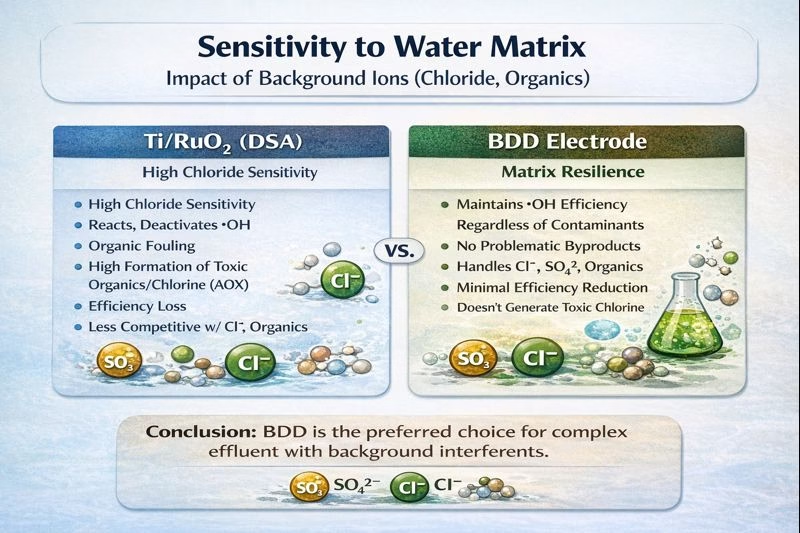
Water Matrixs & Anode Selection
Ti/RuO₂ performance is closely tied to water chemistry. Chloride content, in particular, strongly influences oxidation pathways and efficiency. Changes in waste stream composition can therefore lead to noticeable changes in treatment performance.
Electro oxidation systems adopted BDD electrode as anodic electrode are however mainly more tolerant, as the oxidation process is mainly controlled by hydroxyl radicals rather than mediated species, performance tends to be less sensitive to variations in chloride, sulfate, or ammonia. This robustness is one reason BDD electrode is often chosen for industrial wastewaters with highly variable composition.
When To Choose Ti/RuO2 Anode Or BDD Electrode For Treatment of Real Wastewater
When to choose BDD
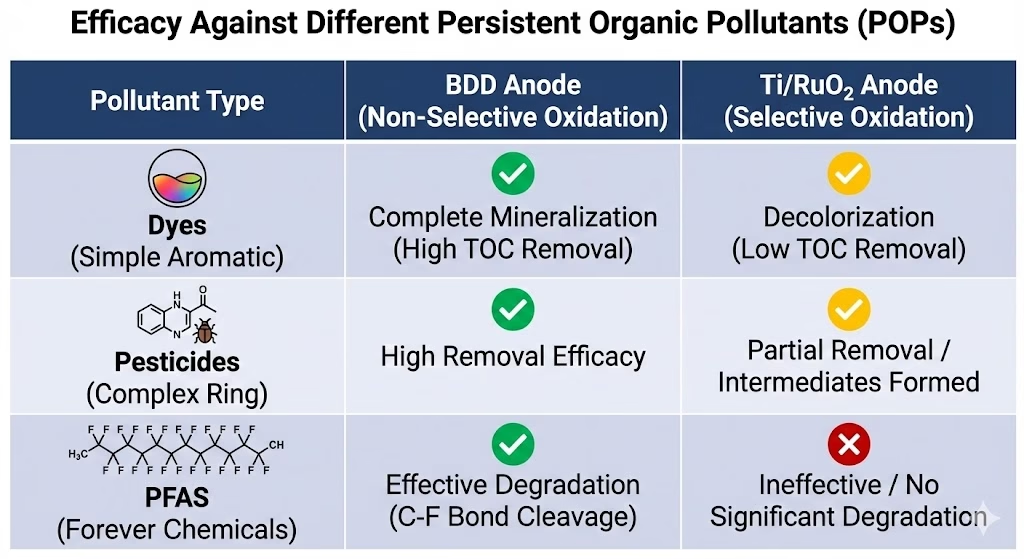
BDD anode is the “next gen” catalyst electro material for refractory wastewater—situations where pollutants are chemically stable or toxic to bacterias.
PFAS & Persistent Organics: BDD is one of the few materials capable of breaking the C-F bond in “forever chemicals.”
Full Mineralization: If the goal is to reduce Total Organic Carbon (TOC) to near zero, BDD electrode’s non-selective hydroxyl radicals cloud is necessary.
Zero Liquid Discharge (ZLD): Ideal for pre-treating concentrated brines before evaporation.
When to choose Ti/RuO2
DSAs like Ti/RuO2 anode are the workhorses for bulk treatment where specific, milder oxidation is sufficient.
Chloride-Rich Wastewater: Ti/RuO2 is an excellent catalyst for the Chlorine Evolution Reaction (ClER). It generates active chlorine species (HClO/ClO-), which are effective for disinfecting municipal water or decolorizing simple dyes.
Selective Modification: If you only need to increase the biodegradability (BOD/COD ratio) of wastewater rather than burning it all to CO2, the lower energy consumption of DSA makes more sense.
A Comparison of Different Electrodes On Hydroxyl Radicals Generation, More Data
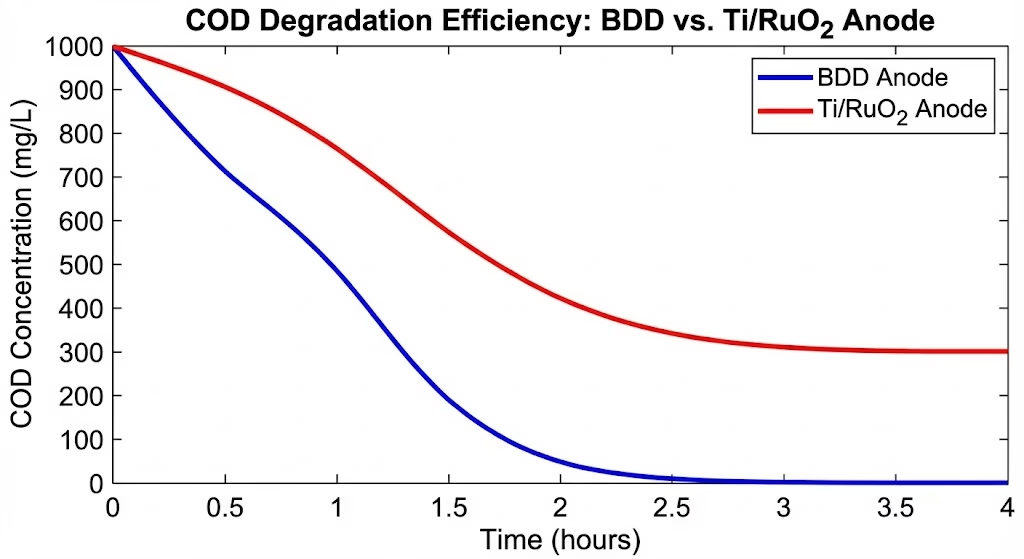
COD removal efficiency and color removal rate help us to get a better understanding of differences between BDD electrodes and conventional electrodes.
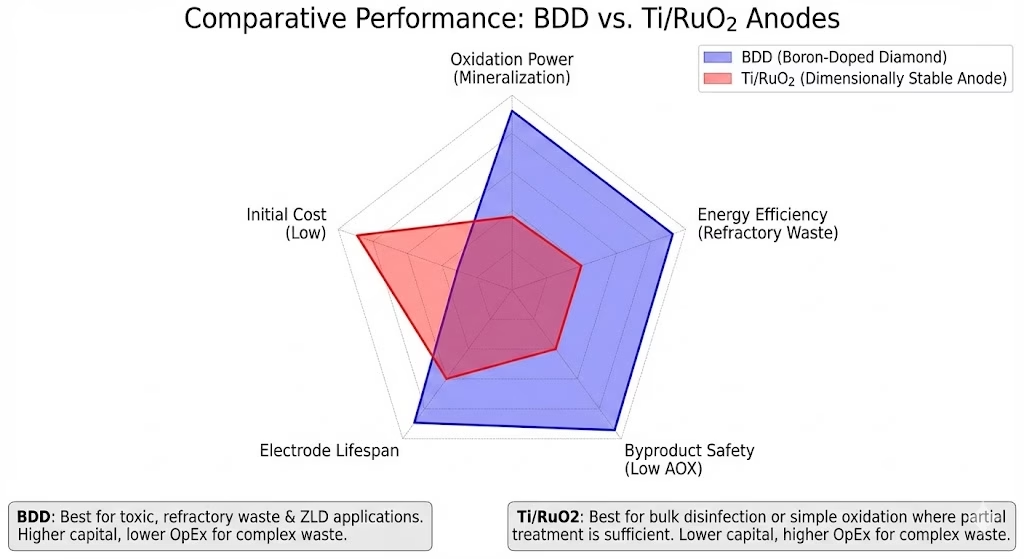
Final Thoughts: A Radar Chart
This radar chart summarizes our last comparison, BDD electrode as in blue polygon excels in oxidation power, energy efficiency for treating refractory waste water, byproduct safety, and electrode lifespan. Its primary drawback is its higher initial cost, we have slashed the cost for posessing BDD electrodes with years of research and development, optimization of CVD processes and operation parameters, increase surface area, tuning boron doping level, testing new substrates
Ti/RuO2 anode red polygon, however it’s a more economical choice upfront but falls short in oxidation capability, efficiency for complex waste, and safety due to potential byproduct formation.
More questions about electrode material selection? reaching out now.
It's strategic decision balancing upfront investment against long-term operational performance and environmental compliance of different electrode materials.
