Electro Oxidation Basics#3: Why BDD Electrode Is Preferred To Iridium Oxide Electrode In EO Process
BDD Electrode Vs. Iridium Oxide Electrode: Hydroxyl Radicals Generation And Electro Oxidation Wastewater Treatment Application
Electro oxidation processes is an efficient and innovative wastewater treatment technology based on advanced oxidaiton processes, for treating various types of complex and refractory industrial and municipal wastewaters. With direct oxidation on the anode surface, and direct oxidation based on the bulk generation of highly reactive species (groups of oxidants degrade and later mineralize organic compounds) at its core, electro oxidation proceses relies on anode material, as surfaces of the anodic electrode is the main spot for highly reactive species generation, major oxidation pathways, e.g. indirect oxidation and direct oxidation, and characteristics of anode materials are matter in energy efficiency, and treatment effectiveness.
Therefore selecting suitable anode material matters in electro oxidation wastewater treatment processes, as phsyi-chemical properties of anode matials affact the efficiency of the generation of highly reactive species, mainly hydroxyl radicals with a reduction/oxidation potential, at 2.73 to 2.80 V vs. SHE, an oxidant with oxidation capability second only to fluorine, •OH radicals can mineralize persistent organic pollutants, therefore affect the overall performance and treatment efficiency of electro oxidation wastewater treatment system.
This specific page is about in-depth comparative analysis of two anode materials: Boron-Doped Diamond BDD electrode and Titanium/Iridium Dioxide electrode (Ti/IrO2), focusing on their apparent mechanisms of hydroxyl radical generation, then applications of these anode materials for electro oxidation wastewater treatment processes, and how does anode electrode material selection and optimizations impact the on-site electro oxidation treatment of wastewater.
Two of the most widely studied and applied anode materials are Boron‑Doped Diamond (BDD) and Titanium‑supported Iridium Oxide (Ti/IrO₂) electrodes. While both are industrially relevant, their fundamental electrochemical behavior is profoundly different—leading to distinct application niches in wastewater treatment.
Radical Generation: The "Active" BDD Electrode vs. "Non-Active" Iridium Oxide Electrode
In this part, we explore hydroxyl radicals generation or say oxidation mechanisms of BDD electrode and Ti/RuO2 anode, dive deeper to figure out the differences between non-active anode and active anode, why some electrode materials perform better than others when it comes to the basics of electro oxidation wastewater treatment processes, which also help you to get a better comprehension about the anode material selection part.
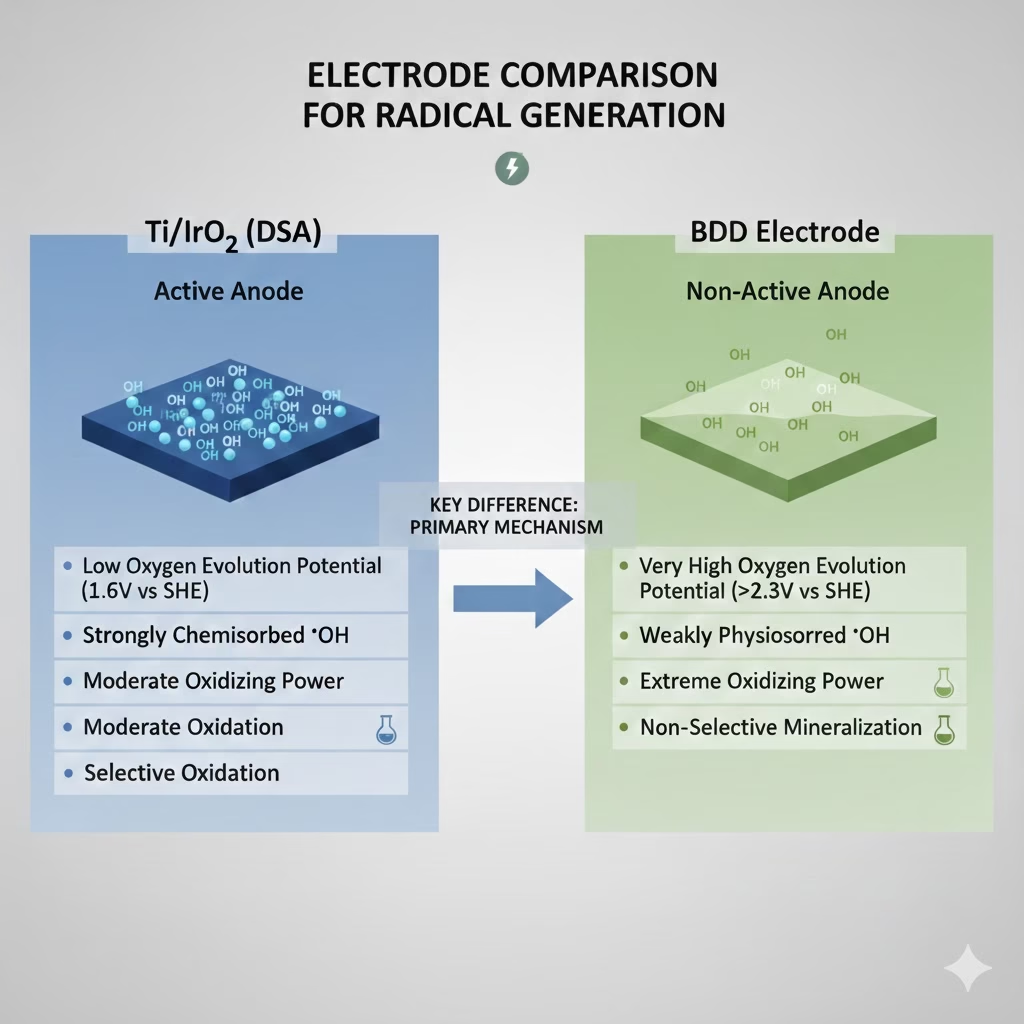
Comparison of BDD Electrode Vs. Iridium Oxide Electrode for Radical Generation
The fundamental difference of reactive species/radicals generation lies in how these electrodes interact with water molecules once potential generated after electricity applied into the electrolytes.
Iridium oxide electrode is highly “active”, functions as catalyst for oxygen evolution reaction which split water to produce oxygen and hydrogen gas rather than high-energy radicals as it has a lower oxygen evolution potential(OEP). Hydroxyl radicals are mainly adsorbed to the surface, forming higher-state oxides. With limited oxidation power, Iridium oxide electrode is better for partial oxidation or “conversion” of organic matters rather than total mineralisation, or green hydrogen gas generation.
BDD electrode is an “non-active” electrode. It has an incredibly high OEP, allows for a weaker interaction between the the anode surface and generated radicals, as they are weakly attached to the anode surface, which expedited reactivity of radicals as well as oxidation of organic pollutants, while suppresses the side reaction of water splitting.
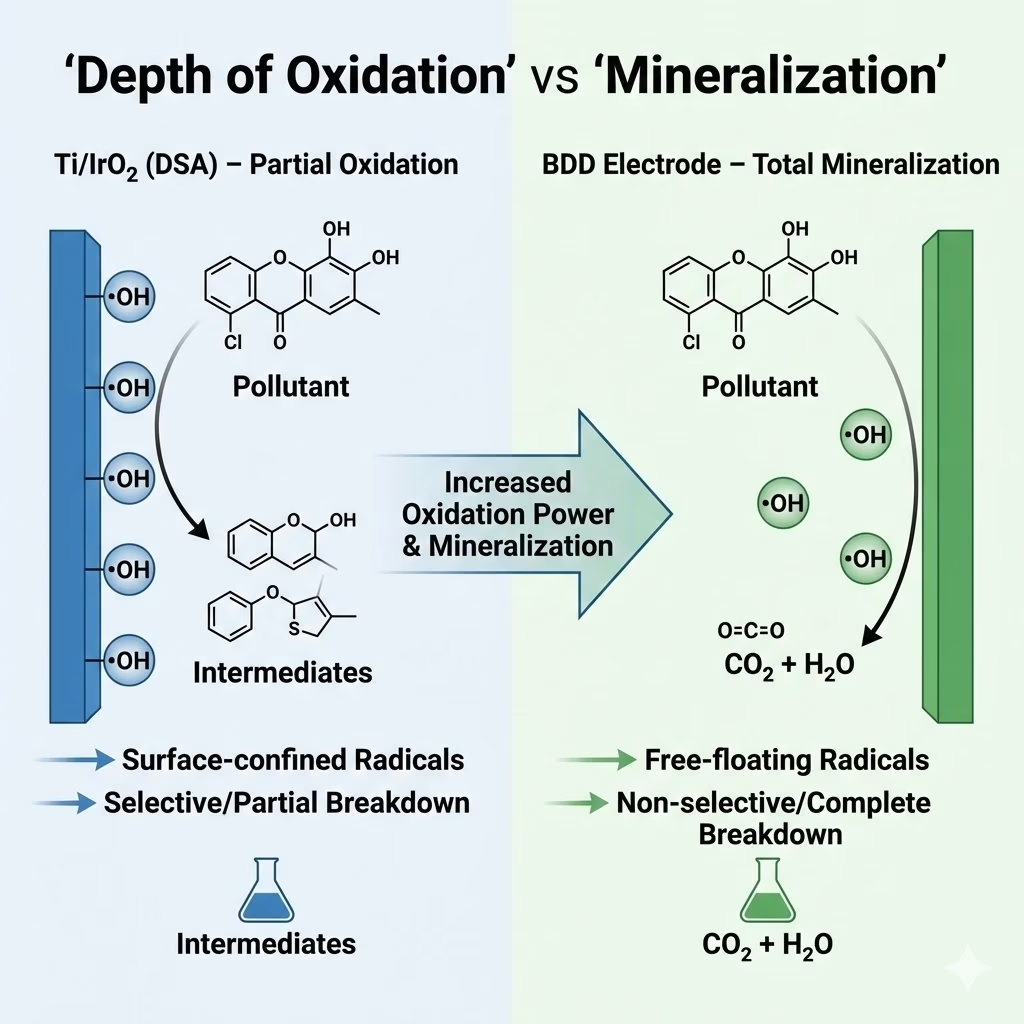
Iridium Oxide Electrode And BDD on Depth of Oxidation and Mineralization
Major difference between Ti/IrO₂ Iridium oxide electrode and BDD electrode lies in chemical breakdown, conversion or mineralization in wastewater treatment.
Partial oxidation or electrochemical conversion of Iridium oxide electrode transforms those organic pollutants into intermediate biodegradable compounds, carboxylic acids and other short-chain intermediates.
Total mineralization or electrochemical combustion of organic pollutants on BDD electrode means organic pollutants are completely destroyed and converted into carbon dioxide, water, and inorganic salts. Persistent organic pollutants can be mineralized even if those wastewaters are very complex, e.g, with high COD concentration, high sanility, highly toxic, high salinity enhances conductivity, reducing energy costs, this process needs no chemical additions, and reduces toxicity, physi-chemical stability of BDD electrode making it robust and emit stable performance in industrial effluent treatment processes.
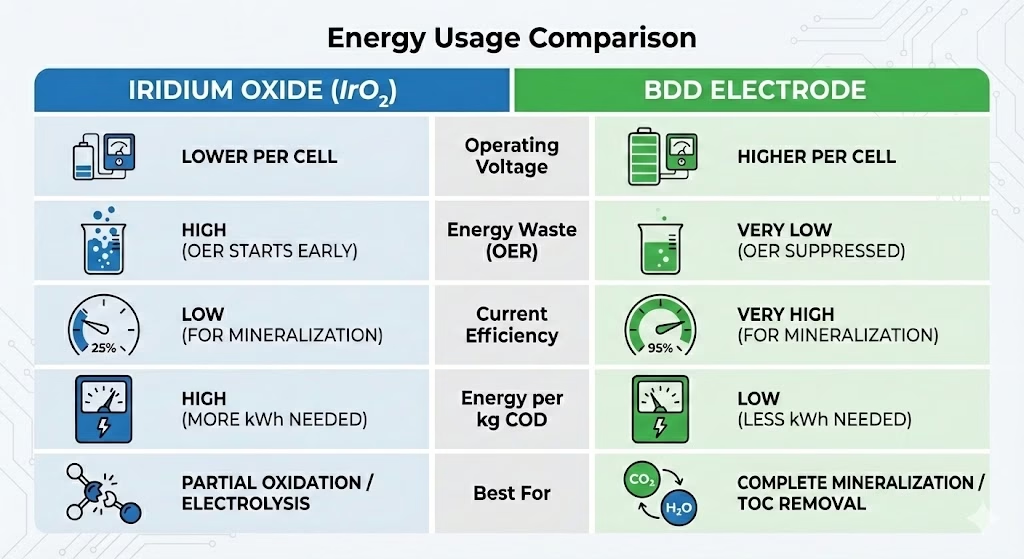
Energy Efficiency Comparison
While Iridium oxide electrode is often cheaper to manufacture, BDD electrodes are significantly more energy-efficient for the complete destruction of organic pollutants. Iridium Oxide IrO2 electrode ,with OEP at ~1.6 V, starts splitting water early, while BDD electrode has OEP over 2.3 V vs. SHE. Wider potential window allows BDD electrode to reach higher energy levels to produce hydroxyl radicals without wasting energy on water splitting. Research indicates that BDD often achieves specific energy consumption rates that are 4 to 8 times lower than IrO2 electrodes for complex wastewater .BDD electrode can maintain near 100% current efficiency in the early stages while IrO2 usually shows below 20–30% for refractory organics removal.
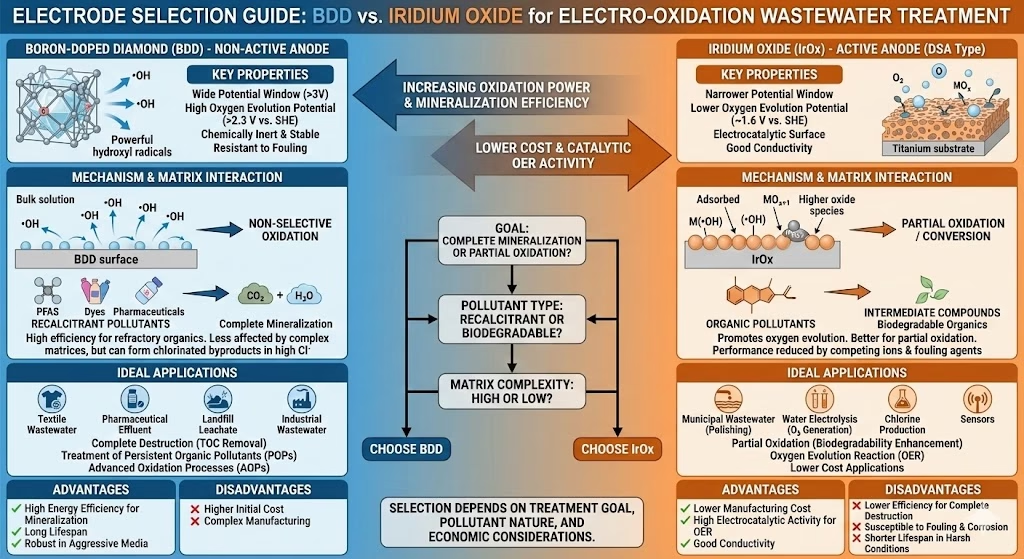
Water Matrixs & Anode Selection
When the water is with high organic load, toxic, complex with persistent organic pollutants. e.g, BTEX, pesticides, or PAH (polycyclic aromatic hydrocarbon), BDD electrode provides the extreme oxidation power required to break down stable carbon chains into carbon dioxide and water, however you need to mitigate byproduct generation.
Selective Iridium oxide electrode if you want to perform disinfection, partial oxidation, enhance biodegradability, or treat waste streams with low organic loads, high chloride, ammonia-laden.
General Organic Loads & Targeted Organic Compounds Removal Efficiency, A Comparison of Ti Iridium Oxide Electrode Vs. BDD Electrode for Treatment of Real Wastewater
Right before we wrapping up all this specific content about comparative comparison of Ti Iridium Oxide electrode and boron doped diamond BDD electrode, let’s take a look at efficacy of these two electrodes toward general organic loads such as COD, and TOC, treatment efficiency toward different types of persistent organic pollutants, it’s a final showdown of oxidation capability and performance in real wastewater treatment.
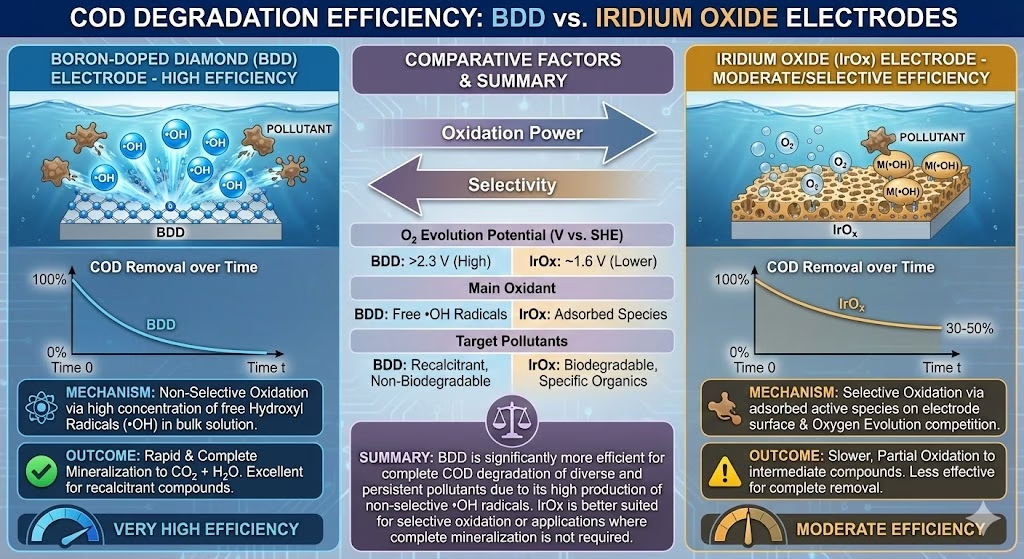
COD degradation efficiency comparison of BDD and Iridium Oxide Electrode
With a high OEP, BDD electrode doesn’t split water into oxygen easily, it creates reactive hydroxyl radicals, weakly adsorbed on the surface, these radicals conduct non-selective attack and break molecules of organic pollutants, while Iridium oxide electrode tends to “convert” those complex organics into intermediates. The COD removal efficiency on BDD electrode reached some 85–96%, while a 60–80% on Iridium oxide electrode.
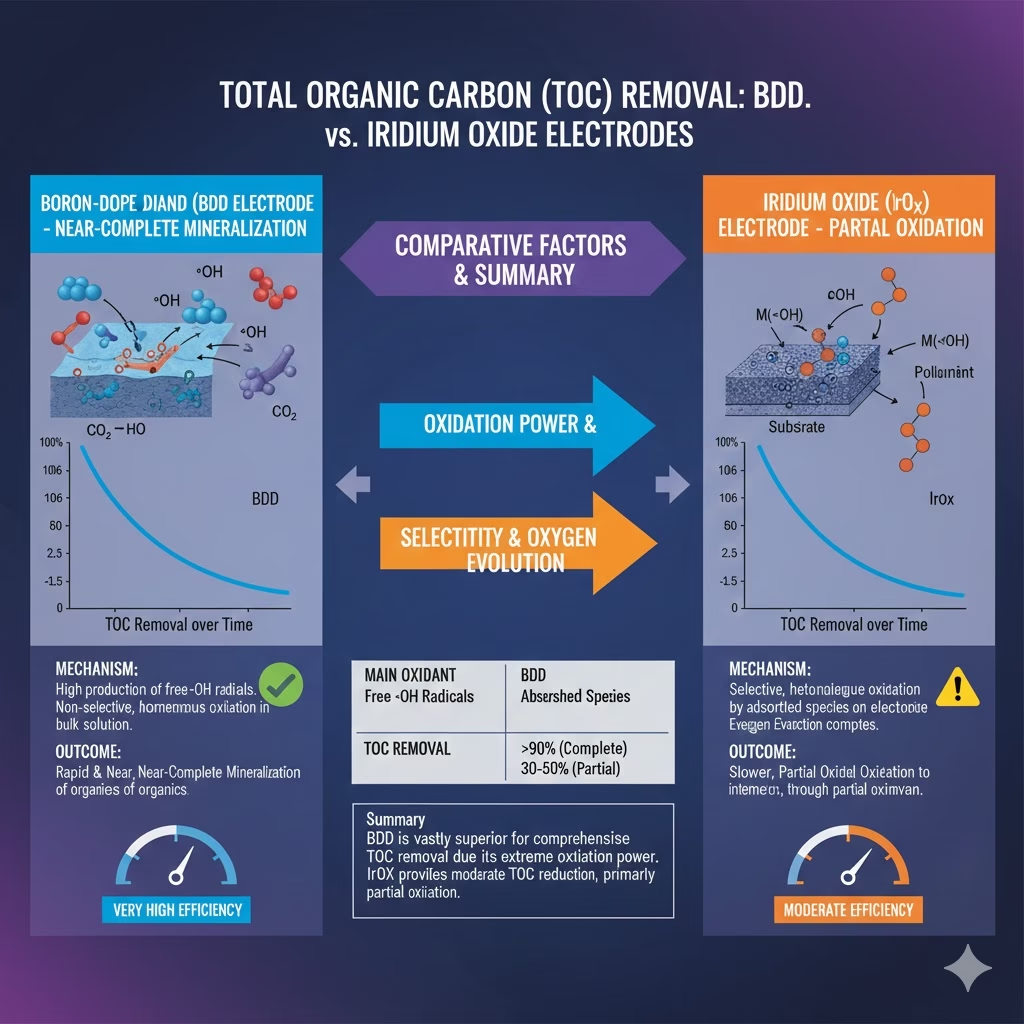
TOC degradation efficiency comparison of BDD and Iridium Oxide Electrode
If COD removal as “cleaning the water,” removing TOC is “deleting the pollutants.” Let’s check what are differences of BDD electrode and Iridium oxide electrode.
The fundamental difference lies in mineralization against conversion when it comes to TOC removal: BDD electrode can break molecular structures of organic carbon, even those aromatic structures such as benzene rings, biphenyls, dioxins, furans, and PAHs, it’s an “incinerator.” it mineralizes organic carbons into carbon dioxide and water, some experiments indicated TOC removal rates at some 80–95% on BDD electrode. Wile Iridium oxide convert organic compounds into various types of intermediates with smaller, simpler molecular structures cab be less toxic or have a lower COD, the carbon is still in the water. It demonstrate decent COD removal efficiency, but some 10-40% TOC removal rate.
Final Thoughts: A Radar Chart
BDD electrode has a very high Oxygen Evolution Potential over 2.3V, and the optimal anode surface allows it to generate free hydroxyl radicals that can break down persistent organic pollutants into CO2, water, and inorganic sales. Iridium Oxide is with a lower OEP at some 1.6V, it starts splitting water into oxygen rather than generate reactive oxidants. It is better for disinfection or enhance biodegradablity of wastewater.
As we mentioned earlier, BDD electrode is some 4 to 8 times more energy-efficient than Iridium oxide when the primary goal is complete degradations of refractory pollutants, as BDD does not spend much energy on “water splitting”, more of the electricity goes directly into carbon removal, that is generation of oxidants. IrO2 can be more efficient for brine electrolysis or chlorine generation.
BDD electrodes are fabricated on substrates like Niobium or Silicon, demonstrate unmatched longevity and can function in some harsh environments for years, however the synthesis process is costy, and there might be toxic byproduct like Perchlorate if there are chloride in the influents. While IrO2 generate Hypochlorite or chlorates, these byproducts are easy to manage, IrO2 is durable, its catalytic coating eventually erodes or “passivates,” demanding some frequent replacing.
Questions about electrode material? Get started to reaching out to us now.
It's strategic decision balancing upfront investment against long-term operational performance and environmental compliance of different electrode materials.
