Electro Oxidation Basics#4: pHOTocatalyst wastewater treatment vs.Electro oxidation Processes
Engineering Advanced Oxidation for Organic Pollutants Via Photocatalytic Wastewater Treatment Processes
Recalcitrant organic pollutants (ROPs) are always a “treatment ceiling” in modern industrial wastewater management processes, these organic compounds with heavier molecular structures designed to persist in regular biological and physical-chemical treatment methods .
Photocatalytic wastewater treatment technology is a premier Advanced Oxidation Process (AOP) that moves beyond simple separation. It doesn’t just move contaminants from the water to a sludge cake; it utilizes molecular-level degradation to mineralize complex organics into harmless byproducts. In this page, we bring in-depth introduction toward photocatalytic processes, the content mainly covers mechanisms, main targeted applications, advantages of photocatalytic in wastewater treatment, then possible challenges in real wastewater treatment, do not miss the content about side-by-side comparison of photocatalytic vs. electro oxidation when it comes to treating complex industrial wastestreams.
Photocatalytic Wastewater Treatment Processes At A Glance
Photocatalytic is an Advanced Oxidation Process (AOP) that uses energy sources from UV or visible lights combined with catalysts, typically semiconductors such as titannium dioxide TiO2 and ZnO to generate highly reactive species in the electrolytes to degrade organic pollutants.
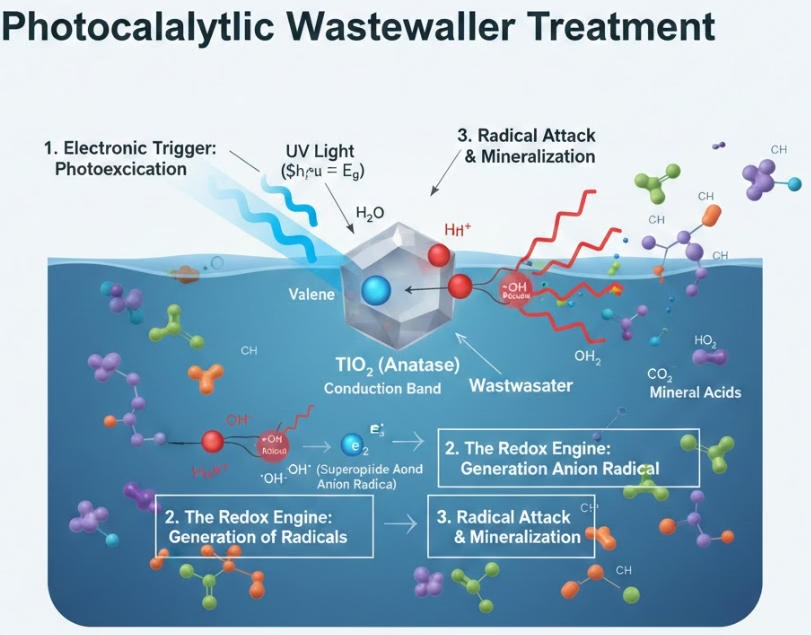
Semiconductor like titanium dioxide has a band gap which is the energy void between the valence band (filled with electrons) and the conduction band (empty). When a photon strikes the catalyst with energy equal to or greater than the band gap (hv ≥ Eg), an electron (e-) is kicked up to the conduction band. This leaves behind a “hole” (h+) in the valence band.
The electron-hole pair is a strengthful redox couple. However, they are fleeting; if they aren’t used immediately, they recombine and release energy as heat, rendering the process useless. These holes are urgently acquiring electrons, they react with water molecules or hydroxide ions adsorbed on the catalyst surface to generate superoxide radicals and hydroxyl radicals, these are among the oxidants with the best powerful oxidation power in known science, that is the oxidation proceses at the Holes (h+) which is in range of surface-level chemistry.
Typical Applications of Photocatalytic Wastewater Treatment Processes
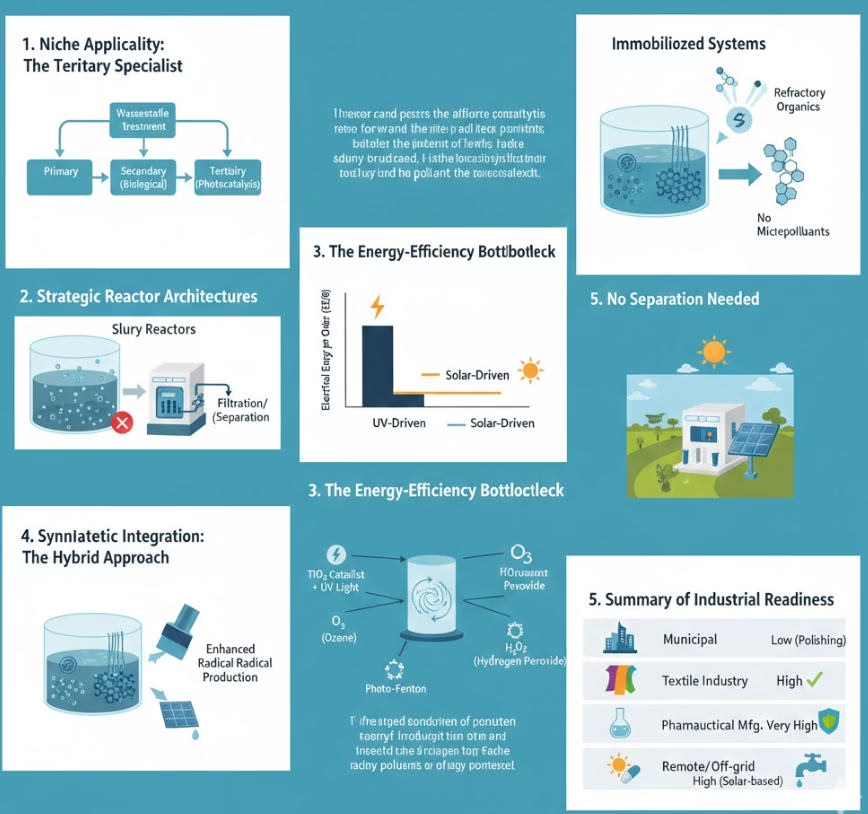
Photocatalysis excels at degrading recalcitrant organic compounds that are refractory to biological systems, e.g, activated sludge can not handle, removing Trace Organic Contaminants (TrOCs), such as endocrine disruptors, pharmaceuticals (e.g., Carbamazepine), and pesticide residues that persist in secondary effluent at ppb levels.
Pure photocatalysis is not viable in modern industrial applications, as catalysts are suspended in the water, UV lamps required frequent replacement, and electric consumptions, therefore hydrogen peroxide based Photo-Fenton or ozone based Photocatalytic Ozonation are introduced to remove organic compounds or micro-plastics from some municipal wastewater, decolorization and degrading complex azo-dyes of effluents from textile industry, direct onsite treatment of toxic pharmaceutical manufacturing effluents. Pathogen inactivation and basic detoxification based on solar system for remote or off-grid area.
Major Advantages of Photocatalytic Wastewater Treatment Processes
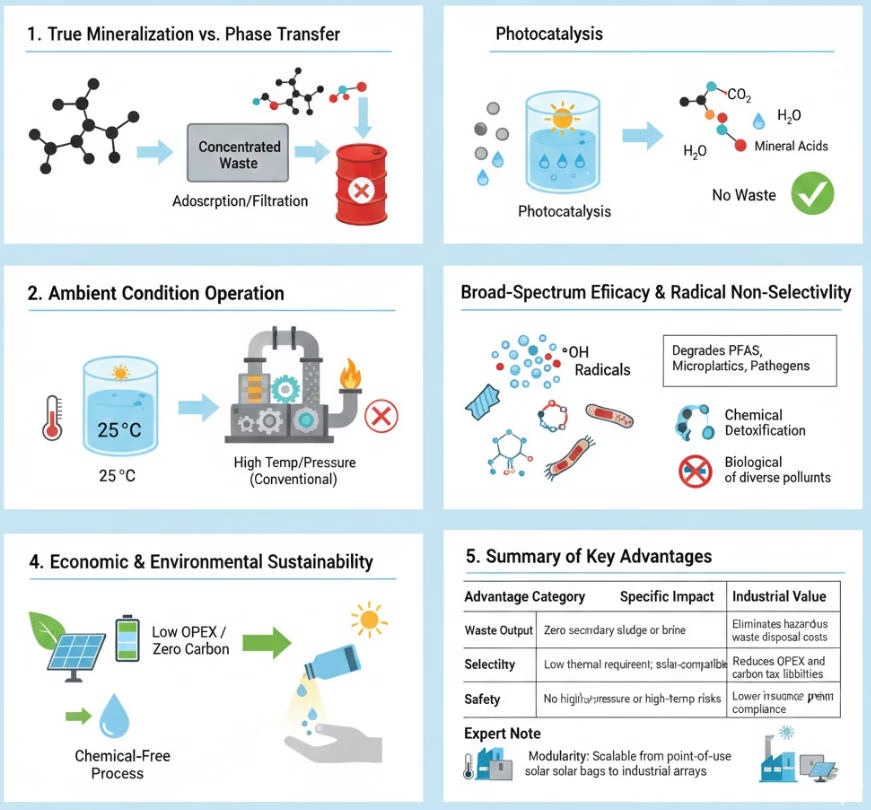
Conventional treatment methods such as adsorption e.g. activated carbon or membrane filtration, e.g. Reverse Osmosis, does not destory organic pollutants, photocatalytic reach complete organic compound removal, there are almost zero secondary sludge or brine.
Thanks to non-selective nature of reactive oxidants generated from the photocatalytic processes, those oxidants attck diverse pollutants from various types of complex waste stream.
Lower thermal requirement and solar-compatiblity slash the OPEX and carbon footprints.
Ambient operation environment, no high-pressure or high-temp risks, lower insurance premiums and simpler compliance, safe to operate.
Major Disadvantages of Photocatalytic Wastewater Treatment Processes
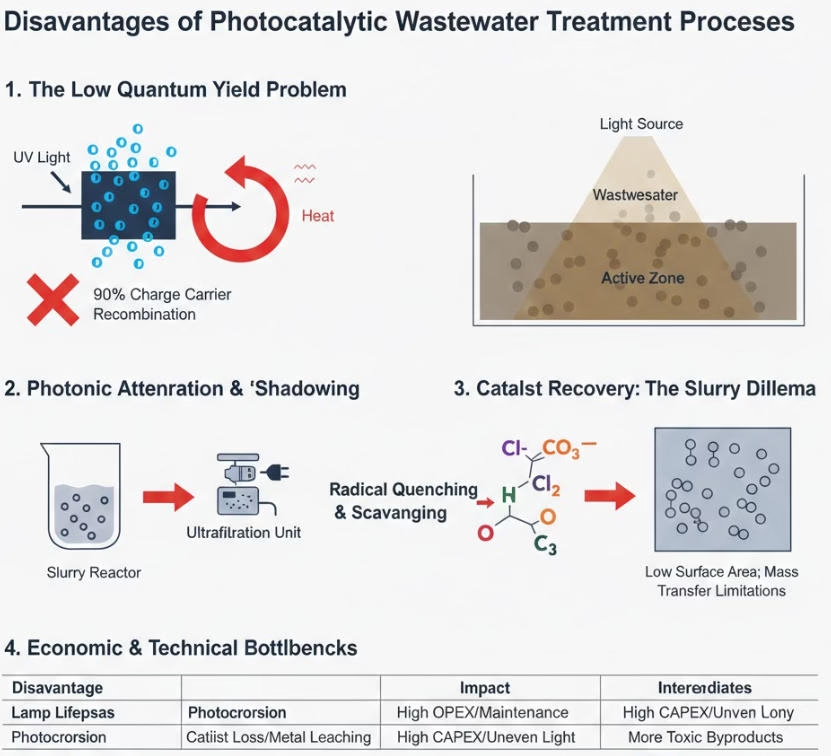
UV lamps degrade and lose intensity over time, therefore lifespan of UV lamps affect the actual implements of photocatalytic, as it requires regular maintenance and replacement, eventually rise OPEX.
Certain catalysts, e.g, ZnO dissolve under light, causing metal leaching/photocorrosion. There will be catalyst replacement costs
Uniform light distribution is nearly impossible in large tanks, which means scaling is complex, high CAPEX for complex reactor geometry.
Partial oxidation can generate intermediates/byproducts more toxic than the original, hence risk of failing toxicity discharge permits.
Heavy dependence on UV lamps and catalysts, inefficient degradation and risk of toxic byproduct generate make photocatalytic challenging.
A Comparative Comparison of Photocatalytic Wastewater Treatment vs. Electro Oxidation Wastewater Treatment Processes for Treatment of Real Wastewater
This sub content is about comparative comparison of photocatalytic wastewater treatment processes vs. electro oxidation processes, hope this content can help you determine which one actually fits you.
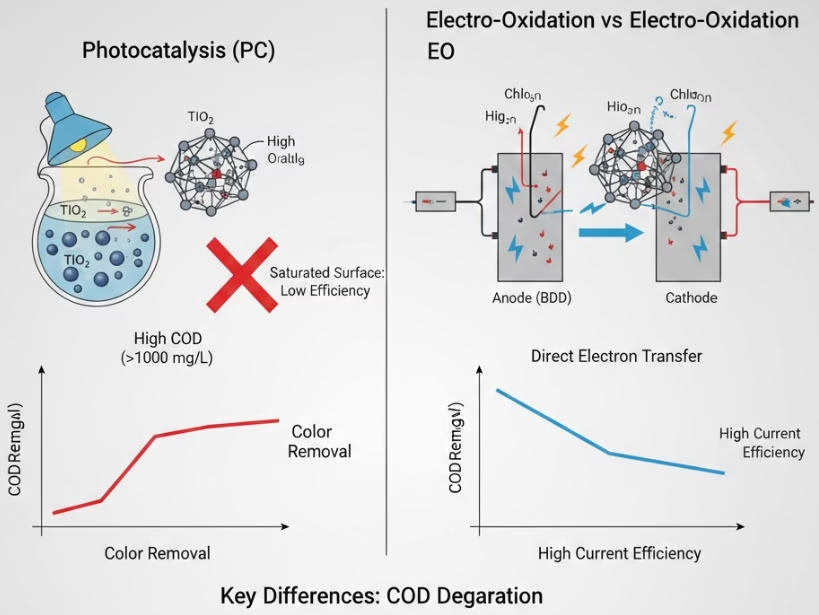
COD degradation efficiency comparison of Photocatalytic vs. Electro Oxidation
Differences between TOC/COD removal, decolorization and mineralization,COD/TOC removal).
When it comes to initial COD removal, photocatalytic is fast for simple aromatics, yet slow for complex chains, electro oxidation is rapid toward most persistent organic pollutants with heavy mocular structures, and proportional to current density (j).
Photocatalytic is low in mineralization rate, stops at intermediate organic acids. Electro oxidation can mineralize organic compounds all the way to CO2 and water.
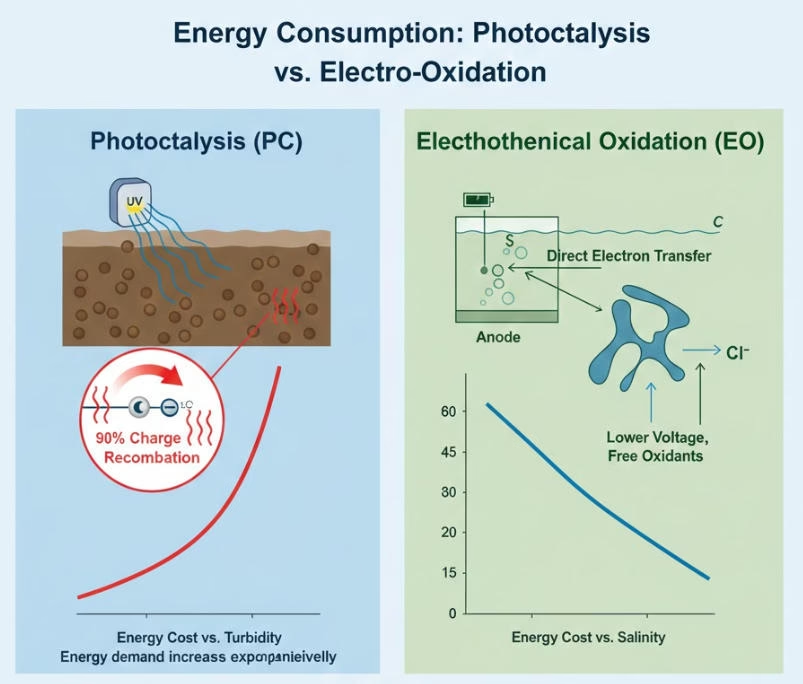
Comparison of Photocatalytic vs. Electro Oxidation on Energy Consumptions
Energy Medium Photons/indirect electricity consumption is the energy medium of Photocatalytic, while electrons/direct electric power is the main source of electro oxidation.
Typical Energy Use Catalytic processes has some over 100 kWh/m3 on Electrical Energy per Order, while electro oxidation records some 1-20 kWh/m3.
Scale-up Impact Energy costs grow with volume when you do the math for scaling-up on Photocatalytic, while electro oxidation reaches stable energy use with efficient modular deployment.
Path to “Green” Both Photocatalytic and electro oxidation can transit to solar and other renewable electricity sources to reach path to green.
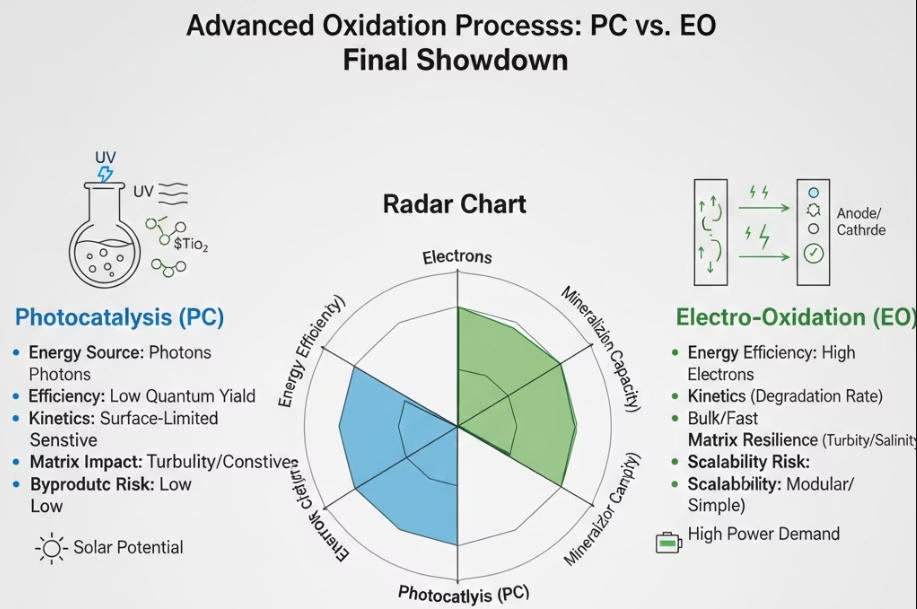
A Final Showdown of Photocatalytic vs. Electro Oxidation Wastewater Processes
For complex waste streams with high-strength COD over 1000 mg/L Electro Oxidation is preferred for its high throughput and direct electron transfer.
Electro oxidation can be adopted to exploit salinity for secondary oxidation of saline industrial brine as high salinity can boost the current density of the electrolytes, one of the main operation parametes.
Photocatalysis is preferred for on-site treating of high sensitivity to ppb-level contaminants for tertiary polishing (Trace TrOCs).
Choose treatment method fits your specific water matrixs and scenario to reach your treatment objectives.
Questions about Photocatalytic? Get started to reaching out to us now.
It's strategic decision balancing upfront investment against long-term operational performance and environmental compliance of different treatment methods.